Nitrate pollution
Nitrate pollution


Nitropollution is the term used for water contamination by nitrate salts mainly used in agriculture. They are responsible for eutrophication in freshwater and marine environments, causing harm to aquatic ecosystems and, consequently, making the survival and reproduction of most forms of life in them significantly difficult. At the same time, drinking water is rendered unfit for consumption/use and it is required it be purified, a costly process.
Protecting the water and the environment from Nitropollution is required by law (106 (1) / 2002), On Controlling Water Contamination.
Nitrate salts are mainly found in fertilizers, manure, compotes, and other organic substances used in or produced by agricultural activity.
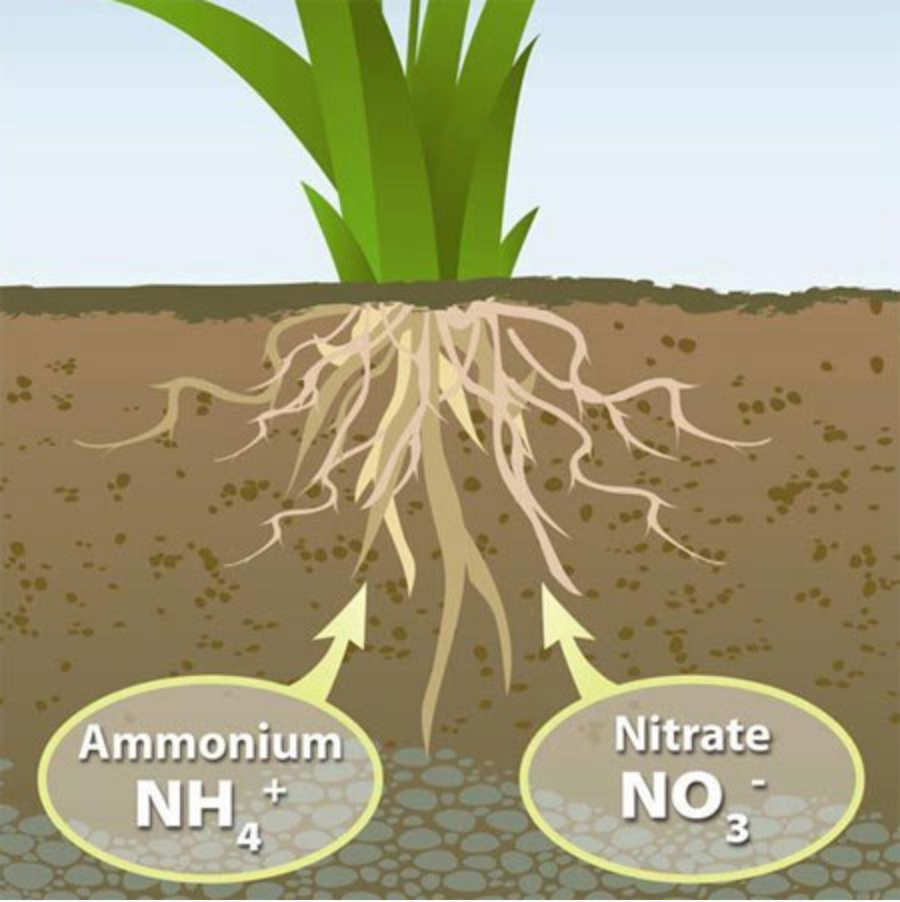
Nitrate ions have high mobility in water, unlike Phosphate and Potassium ions, and they end up in underground waters, contaminating them and rendering them unfit for water supply. Once nitrates exceed the limit of 50 mgr/lt, they can cause health problems and the water must be determined as undrinkable.
In sloping grounds, with soil of low infiltration, nitrates are carried away by surface waters and end up in lakes or the sea, causing eutrophication in aquatic ecosystems.
Proper handling of fertilizers and a sustainable development of agriculture require that industries and users show environmental responsibility and constantly make sure to minimize any potential side-effects which may result from their use. This is possible by making the right plans and predictions so that added Nitrogen inputs in agriculture are in balance with the demands of crops, and that water runoffs and leaching into underground waters are avoided.
During sowing, the bare soil attracts more nitric nitrogen, since there is no retention by the roots, while the demands of plants for Nitrogen during their early stages of development range from very low to minimal.
Therefore, during the basic fertilization of crops, forms of Nitrogen which are gradually absorbed by the plants and are retained in the soil longer should be chosen instead of direct and quickly leached forms such as Nitrates.
From the moment it is absorbed by the soil, Nitric Nitrogen is available to plants for a maximum of 10 days. How long it remains in the zone of the roots depends mostly on rainfall and irrigation of the crops. The higher the rainfall and the greater the quantity of irrigation, the shorter the duration of Nitric Nitrogen being available to plants and the highest its runoff to underground waters.
There are other factors which affect the leaching of Nitrates, such as temperature and type of soil, with high temperatures and sandy soil making runoffs to groundwater easier.
Multiple studies have shown that the Nitric Nitrogen in fertilizers is prone to high losses and is highly responsible for the Nitropollution of aquatic ecosystems.
The main characteristic of this category of fertilizers is longer retention of the applied Nitrogen in the soil and less runoffs to the deeper layers of the ground, which have significant agronomic and environmental benefits.
Stabilized fertilizers significantly reduce Nitrogen losses, allow plants to utilize it more, minimize its leaching to underground waters, and prevent the contamination of groundwater and basins.
The stabilization of Nitrogen in the soil is achieved through absorption during the process of production of special nitrification inhibitors, in both Nitrogen and compound fertilizers.
Nitrification inhibitors are organic molecules of enzyme activity, which slow down the process of nitrification, reduce the speed of Nitrogen splitting, preventing a fast conversion of the Ammoniacal form into Nitric, and prevent its leaching outside the zone of the roots.
By choosing stabilized fertilizers we are not only investing in crop productivity but participating in a more sustainable and friendly development of agriculture, where nitrogen is managed responsibly and the environment is protected for future generations.
Dekastim turbo Micronutrients – NutrActive® extra Micronutrients
One of the most widely known nitrification inhibitors is Dicyandiamide (DCD), which is included in the Stabilized Nitrogen Fertilizers of HELLAGROLIP with the trade name NutrActive®.
NutrActive® fertilizers, through the DCD nitrification inhibitor, stabilize the Ammoniacal Nitrogen in them, protect it from microbial oxidation, and prolong the time it remains in the soil up to 6 to 10 weeks.
By slowing down the conversion rate of Ammoniacal Nitrogen to Nitric, they adjust the supply of Nitrogen based on the needs of the plants, minimize losses due to leaching and vaporization, and maximize the efficiency of fertilization.
Having an ideal ratio between “stabilized” Ammoniacal and Nitric Nitrogen, NutrActive® fertilizers fully cover both the short- and long-term needs of the plants. This way, they manage a prolonged nutrition of the crops with these two absorbable forms of Nitrogen and they protect both underground and surface waters from the risk of Nitropollution.

Contact us!
- For any questions about the products and their use, please free to contact us.
- Tel. : +30 213 003 7600
- Mail : [email protected]
Nitrate pollution

Nitropollution is the term used for water contamination by nitrate salts mainly used in agriculture. They are responsible for eutrophication in freshwater and marine environments, causing harm to aquatic ecosystems and, consequently, making the survival and reproduction of most forms of life in them significantly difficult. At the same time, drinking water is rendered unfit for consumption/use and it is required it be purified, a costly process.
Protecting the water and the environment from Nitropollution is required by law (106 (1) / 2002), On Controlling Water Contamination.
Nitrate salts are mainly found in fertilizers, manure, compotes, and other organic substances used in or produced by agricultural activity.
Nitrate ions have high mobility in water, unlike Phosphate and Potassium ions, and they end up in underground waters, contaminating them and rendering them unfit for water supply. Once nitrates exceed the limit of 50 mgr/lt, they can cause health problems and the water must be determined as undrinkable.
In sloping grounds, with soil of low infiltration, nitrates are carried away by surface waters and end up in lakes or the sea, causing eutrophication in aquatic ecosystems.

Proper handling of fertilizers and a sustainable development of agriculture require that industries and users show environmental responsibility and constantly make sure to minimize any potential side-effects which may result from their use. This is possible by making the right plans and predictions so that added Nitrogen inputs in agriculture are in balance with the demands of crops, and that water runoffs and leaching into underground waters are avoided.
During sowing, the bare soil attracts more nitric nitrogen, since there is no retention by the roots, while the demands of plants for Nitrogen during their early stages of development range from very low to minimal.
Therefore, during the basic fertilization of crops, forms of Nitrogen which are gradually absorbed by the plants and are retained in the soil longer should be chosen instead of direct and quickly leached forms such as Nitrates.
From the moment it is absorbed by the soil, Nitric Nitrogen is available to plants for a maximum of 10 days. How long it remains in the zone of the roots depends mostly on rainfall and irrigation of the crops. The higher the rainfall and the greater the quantity of irrigation, the shorter the duration of Nitric Nitrogen being available to plants and the highest its runoff to underground waters.
There are other factors which affect the leaching of Nitrates, such as temperature and type of soil, with high temperatures and sandy soil making runoffs to groundwater easier.
Multiple studies have shown that the Nitric Nitrogen in fertilizers is prone to high losses and is highly responsible for the Nitropollution of aquatic ecosystems.
The main characteristic of this category of fertilizers is longer retention of the applied Nitrogen in the soil and less runoffs to the deeper layers of the ground, which have significant agronomic and environmental benefits.
Stabilized fertilizers significantly reduce Nitrogen losses, allow plants to utilize it more, minimize its leaching to underground waters, and prevent the contamination of groundwater and basins.
The stabilization of Nitrogen in the soil is achieved through absorption during the process of production of special nitrification inhibitors, in both Nitrogen and compound fertilizers.
Nitrification inhibitors are organic molecules of enzyme activity, which slow down the process of nitrification, reduce the speed of Nitrogen splitting, preventing a fast conversion of the Ammoniacal form into Nitric, and prevent its leaching outside the zone of the roots.
By choosing stabilized fertilizers we are not only investing in crop productivity but participating in a more sustainable and friendly development of agriculture, where nitrogen is managed responsibly and the environment is protected for future generations.
Dekastim turbo Micronutrients – NutrActive® extra Micronutrients
One of the most widely known nitrification inhibitors is Dicyandiamide (DCD), which is included in the Stabilized Nitrogen Fertilizers of HELLAGROLIP with the trade name NutrActive®.
NutrActive® fertilizers, through the DCD nitrification inhibitor, stabilize the Ammoniacal Nitrogen in them, protect it from microbial oxidation, and prolong the time it remains in the soil up to 6 to 10 weeks.
By slowing down the conversion rate of Ammoniacal Nitrogen to Nitric, they adjust the supply of Nitrogen based on the needs of the plants, minimize losses due to leaching and vaporization, and maximize the efficiency of fertilization.
Having an ideal ratio between “stabilized” Ammoniacal and Nitric Nitrogen, NutrActive® fertilizers fully cover both the short- and long-term needs of the plants. This way, they manage a prolonged nutrition of the crops with these two absorbable forms of Nitrogen and they protect both underground and surface waters from the risk of Nitropollution.


